Understanding Steel Wire Corrosion in Underground Mines: Risks, Causes, and Prevention
Corrosion of high-carbon steel wires, especially those used in cablebolts for underground mining, poses a significant long-term threat to structural integrity and worker safety. In deep, damp, or chemically aggressive environments—like acidic or saline groundwater—cables are constantly exposed to conditions that promote degradation.
 |
| Image: Corroded steel strand used in underground mining cables. |
How Corrosion Works
At a basic level, corrosion is an electrochemical reaction involving:
-
Anodic reaction (metal loses electrons)
-
Cathodic reaction (electrons are accepted)
In the case of steel, iron atoms release electrons and form compounds such as iron oxide (rust) or iron hydroxide. These processes are often triggered by the presence of:
-
Water
-
Oxygen
-
Acid or salt (electrolytes)
Corrosion in mines is accelerated by humidity, cracks, temperature, and chemical reactions in groundwater.
Types of Corrosion in Steel Cables
1. Dry Corrosion
Occurs slowly during storage or exposure to dry air. It forms a thin iron oxide layer that may look harmless but can spread.
-
Usually triggered by high temperatures or long sun exposure.
-
May reduce cable strength by up to 20% (Goris, 1990).
2. Wet or Atmospheric Corrosion
Occurs rapidly in humid, wet, or underground environments.
-
Produces softer, greasy rust like magnetite (Fe₃O₄) or hematite (Fe₂O₃).
-
Weakens the bond between grout and cable, reducing tensile strength.
-
Leads to brittle failures, cracks, and long-term instability.
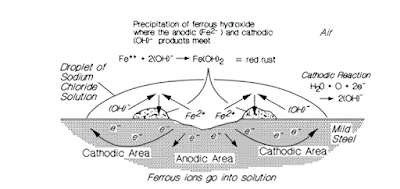
Galvanic Corrosion and Electrolytes
When steel is exposed to chlorides, sulfates, or acidic water, corrosion becomes galvanic:
-
Anodes (damaged steel) and cathodes (wet zones) create electric currents inside the cable.
-
Chloride-rich mine water creates continuous electrochemical cycles that eat away the steel.
-
Eventually, grout bonding fails, compromising the overall support system.
Preventive Measures
To reduce corrosion risk:
-
Use corrosion-resistant coatings or galvanized cables.
-
Store cables in dry, shaded environments.
-
Improve mine drainage and sealing to reduce exposure.
-
Monitor electrolyte concentrations in water.
Sources:
-
Illston et al., 1979; Pohlman, 1987
-
Minick and Olson, 1987
-
Goris, 1990
-
Stillborg (Rock Support in Mining)

